Category: Uncategorized
10 minutes read
NHS Value-Based Procurement Delay: A Call for Urgent Action and Strategic Innovation
Following recent discussions on the NHS’s efforts to adopt a value-based procurement (VBP) methodology, it’s clear there is room for reflection and concerted action. As noted by Chris Whitehouse , a month has passed since the announcement of a new VBP approach, which aims to place greater emphasis on patient outcomes and experiences in purchasing decisions. However, the comprehensive toolkit to guide this methodology is still pending release. This delay presents a challenge to improving the NHS’s procurement efficiency, an issue that has led to considerable financial implications under the current system.
The proposed VBP methodology represents a significant shift towards prioritising the overall value that products and services provide to patient care, moving away from a focus solely on cost. This approach is designed to enhance healthcare delivery throughout England by ensuring that procurement decisions directly support patient care and contribute to system-wide efficiency.
Transitioning to a VBP model, however, has encountered some hurdles due to existing procurement practices within the NHS, which have historically emphasised cost over value. This has impacted potential savings and the quality of patient care, as seen in the procurement of absorbent continence products. Despite evidence supporting a value-based approach, traditional procurement practices have not always aligned with optimal patient experiences and outcomes.
The delay in rolling out VBP highlights the importance of ongoing commitment to innovation and patient-focused care within the NHS. Given the broad political support for VBP, addressing these challenges is critical for enhancing healthcare procurement and delivery.

Vamstar suggests a strategic revision of NHS procurement practices through the use of healthcare procurement technologies supported by artificial intelligence. By employing advanced data analytics and market intelligence, the NHS can make more informed decisions that adhere to VBP principles. This strategy includes utilising predictive analytics for assessing the long-term impact of procurement choices, incorporating patient feedback into procurement decisions, and building relationships with suppliers dedicated to innovation and quality.
Furthermore, the NHS should foster a culture of innovation within its procurement teams, encouraging purchasing managers to value overall impact over immediate cost savings and to consider the effects of decisions on patient care and the sustainability of the health system.
Vamstar is committed to enriching the knowledge of healthcare procurers by introducing a content-rich platform focused on exploring innovative procurement methodologies and the advantages of advanced data analytics.
The move towards value-based procurement is an essential step in evolving healthcare delivery. With the adoption of innovative procurement solutions and technologies, the NHS is well-positioned to achieve its goals of providing superior, patient-centered care. The current delay in implementing VBP serves as a reminder of the need for all stakeholders in healthcare procurement to intensify their efforts and ensure the NHS’s capabilities to deliver quality care promptly.
For those interested in learning more about Vamstar and its offerings, our team is ready to provide detailed information and discuss how we can support your procurement needs. Further contact details are available below.
Other Materials
Book a 30 minutes meeting with us
Welcome to our scheduling page! Please choose an available date below to get started.
30 minutes meeting
We’ll email you the meeting link
3 minutes read
Rising Importance of Global Health Initiatives in the Pharmaceutical Industry: Trends, Challenges, and Opportunities
Leading pharmaceutical companies are increasingly focusing on global health initiatives, which has led to a rise in procurement through tenders and long-term agreements (LTAs) by Intergovernmental Organizations (IGOs) and Non-Governmental Organizations (NGOs).
In 2023, the total value of pharmaceuticals and medical supplies distributed by these entities is estimated at US$ 21.3 billion, with a significant portion allocated to vaccines, medical supplies, and diagnostics. Pharmaceuticals alone account for about US$ 4.7 billion of this total.
The Covid-19 pandemic caused a noticeable surge in healthcare spending by NGOs and IGOs in 2021, particularly in diagnostics and vaccines. Although the pharmaceutical sector experienced a decline during this period, it has been witnessing a notable upward trend since 2022, with an average year-over-year growth of over 11%.
This trend underscores the growing significance of global health teams within pharmaceutical companies.
These teams are becoming increasingly important due to various factors:
1. Shifting Disease Landscape: There’s a growing prevalence of neglected tropical diseases and infectious diseases in low- and middle-income countries (LMICs), requiring innovative approaches and partnerships for drug development.
2. Equity and Access: Heightened awareness of healthcare inequities in LMICs is pressuring pharmaceutical companies to improve access to essential medicines. Global health teams are pivotal in addressing these challenges by developing sustainable distribution models and navigating complex healthcare systems.
3. Emerging Markets: LMICs are emerging as significant markets for pharmaceuticals. Global health teams are key in helping companies understand and penetrate these markets.
4. Pandemic Preparedness: The COVID-19 pandemic highlighted the need for improved outbreak preparedness. Teams with expertise in public health and vaccine development are valuable for future pandemic response strategies.
5. Regulatory Landscape: As governments and international bodies implement policies to enhance medicine accessibility in LMICs, global health teams are crucial for ensuring compliance and navigating these regulations.
Companies are recognising that strong global health programs not only fulfill social responsibilities but also attract consumers and investors. These programs are focused on making medicines more affordable and accessible in LMICs through various initiatives.
Industry Response Includes:
- Establishing dedicated global health units.
- Engaging in public-private partnerships.
- Exploring innovative financing models.
- Investing in local capacity building.
- Developing medicines for neglected tropical diseases (NTDs).
Challenges and Opportunities:
Despite their importance, global health teams face challenges such as limited resources and the need to balance commercial objectives with social impact. However, the opportunities for significant social impact, market access, and long-term value creation are substantial. These teams play a crucial role in strengthening healthcare systems, advocating for policy change, and demonstrating the value of global health initiatives.
One major challenge is the lack of transparency and market intelligence in the sector, where a large portion of the market remains non-tender based and procurement is often local and in partnership with governments. Vamstar is addressing this challenge by combining AI-based data scraping with subject matter expertise to provide ATC class-level market insights, which are vital for shaping global health strategies in the pharmaceutical industry.
Speak To Our Team Today
We are here to help your team navigate the market efficiently and enhance your market success. Contact us now to schedule a consultation and see how we can assist you in achieving a competitive advantage.
Other Materials
Book a 30 minutes meeting with us
Welcome to our scheduling page! Please choose an available date below to get started.
30 minutes meeting
We’ll email you the meeting link
3 minutes read
Global Interventional Cardiology Market Report Preview (2024 Update)
The realm of interventional cardiology (IC) stands at the cusp of a transformative era, marked by rapid technological advancements, evolving clinical practices, and an ever-expanding scope of therapeutic applications. The Global Interventional Cardiology Market Report offers an unparalleled deep dive into this dynamic field.
This exhaustive analysis covers the spectrum of IC techniques, devices, market trends, and growth prospects, positioning itself as an indispensable resource for a diverse audience including medical professionals, commercial teams, researchers, investors, and stakeholders.
The report draws upon a rich tapestry of sources to ensure a robust and comprehensive market analysis. Engagements with key stakeholders across the spectrum, ranging from IC device suppliers and policy experts to clinicians and hospital executives — provide foundational insights.
The research is further bolstered by an analysis of financial reports, product brochures, technical literature, and a decade’s worth of in-house data leverage through Vamstar’s AI, ensuring a multi-faceted perspective on the industry.
1. Comprehensive Market Overview: Provides a detailed examination of the interventional cardiology (IC) market, including an extensive review of IC techniques, devices, and current market trends. This feature ensures readers gain a thorough understanding of the field.
2. In-depth Analysis of Devices: Each device category, from aspiration catheters to vascular closure devices, is analysed to offer insights into their applications, technological advancements, and market positioning.
3. Robust Research Methodology: Compiled using a mix of primary interviews with industry stakeholders and secondary research, including financial reports, public sources, and technical literature, ensuring a well-rounded perspective on the IC market.
4. Detailed Forecasting: Utilises a dual approach combining ‘bottom-up’ and ‘top-down’ analyses for market estimates, offering precise forecasts from 2024 to 2028. This predictive insight is crucial for strategic planning and investment decisions.
5. Global Coverage with Regional Insights: Segments the market into key geographical regions, providing specific insights into North America, South America, Europe, the Middle East and Africa, and Asia Pacific. This allows for a granular understanding of regional dynamics and opportunities.
This report is designed to be an essential tool for anyone looking to navigate the complexities of the interventional cardiology market, offering both a broad overview and detailed insights to support a wide range of strategic objectives.
1. Strategic Decision Support: Empowers medical professionals, commercial teams, investors, and stakeholders with actionable insights and comprehensive market intelligence for informed decision-making.
2. Commercial Guidance: Offers investors and commercial teams data-driven insights into growth prospects, technological trends, and market dynamics, aiding in the identification of lucrative investment opportunities and potential areas for expansion.
3. Competitive Edge: Equips device manufacturers and suppliers with a detailed understanding of the competitive landscape, helping them to strategise effectively and stay ahead in the market.
4. Policy and Procurement Planning: Assists policy experts, procurement specialists, and hospital executives in understanding current trends and future directions of the IC market, facilitating better planning and procurement strategies.
5. Educational Value: Serves as a valuable educational resource for researchers, clinicians, and medical professionals seeking to deepen their knowledge of interventional cardiology, its techniques, and its devices.
The Global Interventional Cardiology Market Report emerges as a beacon for those navigating the complex waters of the IC industry. Its in-depth analysis, comprehensive market insights, and forward-looking projections equip stakeholders with the knowledge to make informed decisions, harness opportunities, and face challenges head-on in this rapidly evolving field.
Go to Vamstar.io to find out more!
Other Materials
Book a 30 minutes meeting with us
Welcome to our scheduling page! Please choose an available date below to get started.
30 minutes meeting
We’ll email you the meeting link
5 minutes read
Shaping Your Key Account Management (KAM) Strategy using Environmental, Social, and Governance (ESG) in the European MedTech Sector

The European medical technology (MedTech) landscape is undergoing a significant shift. ESG factors are no longer just buzzwords; they’re rapidly becoming essential considerations for key account management (KAM) strategies. Hospitals, healthcare providers, and other key accounts are increasingly prioritising partnerships with MedTech companies that demonstrate strong ESG performance.
A growing number of European healthcare institutions are embedding ESG criteria into their procurement decisions. A Bain & Company survey reveals that 82% of German healthcare procurement decision-makers expect ESG to become even more important in the next five years.
Vamstar’s recent study on the usage of ESG criteria in MedTech tenders across Europe highlighted the increase in usage of mandatory and value-adding criteria across Europe. One of the observations was inherent inconsistencies in objectives, criteria, and language.
The swift integration and diversification of ESG clauses by buyers present a formidable challenge in assessing associated risks. The lack of uniformity in criteria and language employed by various buyers, even within a single province, poses a significant hurdle for suppliers. Buyers exhibit diverse focal points concerning E, S and G aspects, as well as product domains dictated by their consumption patterns and local regulatory frameworks. This divergence results in a lack of standardisation in criteria among buyers, thereby complicating the evaluation process.
Organisations exhibit substantial diversity in their objectives, each emphasising distinct focus areas such as carbon footprint reduction, waste management, product durability, social inclusivity, combatting child labour, and safeguarding data privacy. Furthermore, there exists a notable deficiency in buyers’ comprehension regarding the methods to ensure adherence to ESG criteria. Consequently, suppliers encounter challenges in providing requisite information consistently due to the varied formats and structures sought by buyers.
The exponential surge in the utilisation of ESG criteria, particularly those pertaining to environmental concerns, across countries, has escalated the risk of revenue loss. This trend is anticipated to persist and intensify until 2026, particularly across Europe.
The utilisation of criteria, language, and requisites varies not only across countries or regions but also among individual buyers. Consequently, there arises a necessity for tracking intelligence pertinent to all key accounts in order to discern prevailing trends.
Suppliers must align their key account management with ESG requirements to enhance risk management and cultivate a product differentiation strategy. Given that in most markets, a small number of key accounts contribute significantly to revenue generation, any alteration in ESG policy poses both risks and opportunities. An ESG tracker emerges as imperative, furnishing granular insights into evolving purchasing prerequisites.
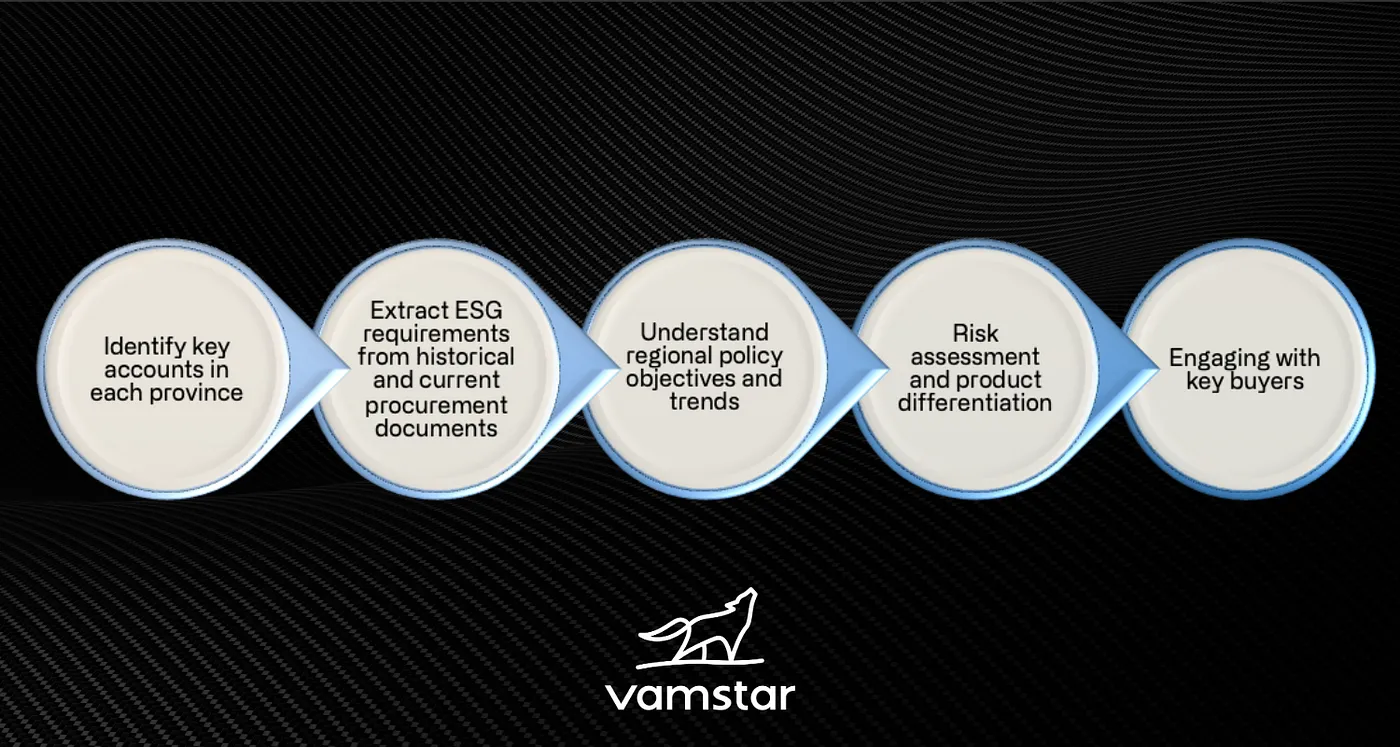
In the MedTech sector, where innovation intersects with human health and well-being, ESG considerations hold profound significance. Integrating ESG into Key Account Management Strategies would result in the following advantages:
1. Building Trust and Credibility: Incorporating ESG principles into KAM strategies fosters trust and credibility with key accounts. Aligning buyer’s ESG objectives with the This resonates well with key stakeholders, including healthcare providers, institutions, and regulatory bodies.
2. Risk Mitigation and Compliance: ESG integration in KAM strategies enables companies to identify and mitigate potential risks more effectively. By proactively addressing environmental risks, such as pollution or resource depletion, companies can avoid regulatory fines and reputational damage.
3. Driving Innovation and Differentiation: Embracing ESG considerations within KAM strategies stimulates innovation and differentiation within the MedTech sector.
4. Enhancing Long-Term Relationships: Successful KAM strategies are built on long-term, mutually beneficial relationships with key accounts. By integrating ESG principles into these relationships, companies can deepen engagement and foster loyalty among key stakeholders.
5. Cost Reduction: Sustainable practices can lead to cost savings through reduced waste, energy efficiency, and responsible sourcing.
Conclusion
In the dynamic European MedTech landscape, integrating ESG into your KAM strategy is no longer optional. It’s a strategic imperative for building long-term, sustainable partnerships with key accounts. By understanding your key accounts’ priorities, aligning your efforts with their needs, and building a data-driven ESG narrative, MedTech companies can solidify their position in a rapidly evolving market.
Other Materials
Book a 30 minutes meeting with us
Welcome to our scheduling page! Please choose an available date below to get started.
30 minutes meeting
We’ll email you the meeting link
3 minutes read
Top 50 use-cases of AI for the commercial teams in the MedTech and the Pharma Industry

AI utilisation is growing in the MedTech and Pharmaceutical sectors as companies aim to boost their operating margins amidst economic challenges. The future victors will use AI to enhance performance and pinpoint trends and signals that will mould upcoming markets. Plus, numerous pilot projects are underway across the industry. Over the past three years, we’ve held over a thousand discussions and carried out multiple implementations / projects of our AI platform in tendering, pricing, market access, and sales.
Below are the top 50 use-cases (alphabetically organised):
- Automated Reporting: Generate sales reports automatically.
- Bid Success Rate Prediction: Estimate the likelihood of winning a tender.
- Competitor Analysis: Monitor and analyse competitor strategies.
- Cost-Effectiveness Analysis: Evaluate the cost-effectiveness of products.
- Demand Forecasting: Predict demand based on tender data.
- Discount Analysis: Assess the impact of discounts on profitability.
- Document Automation: Auto-generate tender documents.
- E-commerce Integration: AI-driven online sales platforms and ERP data harmonisation.
- Historical Data Analysis: Analyse past tender data for patterns.
- Historical Pricing Trends: Analyse past pricing data for insights.
- Inventory Management: Predict inventory needs and shortages.
- Lead Scoring: Prioritise leads based on data-driven insights.
- Licensing Pricing Analysis: Set prices for licensing deals.
- Margin Analysis: Analyse profit margins on tender bids.
- Market Entry Strategy: Analyse the best routes for entering new markets.
- Market Growth Predictions: Predict potential market growth rates.
- Market Segmentation: Segment markets for targeted approaches.
- Market Share Analysis: Based on won and lost tenders.
- Market Trend Analysis: Predict and adapt to emerging market trends.
- Multichannel Marketing: Coordinate campaigns across channels.
- New Product Pricing Analysis: Determine prices for new product launches.
- Order Management: Automate processing of customer orders.
- Patient Access Analysis: Understand patient accessibility to products.
- Policy Impact Analysis: Predict impacts of health policies.
- Population Health Analysis: Understand health trends in populations.
- Post-Market Surveillance: Monitor products post-launch.
- Predictive Analytics for Pricing: Predict future pricing outcomes.
- Price Anomaly Detection: Detect and correct pricing errors.
- Price Optimisation Algorithms: Develop algorithms for automated pricing decisions.
- Price Segmentation: Set different prices for different customer segments.
- Pricing A/B Testing: Test different prices to determine the best strategy.
- Pricing Contract Analysis: Analyse and optimise pricing contracts.
- Pricing Simulation: Simulate pricing scenarios to predict outcomes.
- Pricing Strategy Optimisation: Determine optimal bid pricing.
- Product Recommendations: For sales reps during client meetings.
- Quote Management: Automated quote generation.
- Real-time Analytics: Provide real-time sales data.
- Real-time Tender Data Analysis: Real-time insights during bidding.
- Rebate Analysis: Evaluate the impact of rebates on overall pricing strategy.
- Sales Playbooks: AI-generated sales strategies.
- Supply Chain Impact on Pricing: Analyse how supply chain factors affect pricing.
- Tender Alert Systems: Notify teams of relevant tenders.
- Tender Data Cleansing and CRM Management: Cleanse and refine tender data.
- Tender Data Visualisation: Visual tools for tender data.
- Tender Financial Modelling: Model financial aspects of tenders.
- Tender Market Analysis: Analyse specific tender markets.
- Tender Outcome Predictions: Predict the outcome of tender submissions.
- Tender Prediction: Predict upcoming tender opportunities.
- Tender Reporting: Automated generation of tender reports.
- Value-based Pricing: Price products based on perceived value.
Other Materials
Book a 30 minutes meeting with us
Welcome to our scheduling page! Please choose an available date below to get started.
30 minutes meeting
We’ll email you the meeting link