Category: Uncategorized
6 minutes read
Revolutionising Healthcare in Europe: A Path to Resilience and Innovation

Europe is at the forefront of a transformative journey in healthcare. Home to over 447 million people across 27 diverse countries, the European Union (EU) is tackling the monumental task of providing high-quality healthcare in the face of aging populations, chronic diseases, and the pressing need for medical innovation. This blog delves into the myriad of strategies and initiatives that are paving the way for a future-proof lifescience systems, one that not only addresses today’s challenges but also anticipates the needs of tomorrow.
A Vision for the Future: Horizon Europe and Beyond
At the heart of Europe’s push for healthcare innovation were the Horizon 2020 and Horizon Europe programs. Although Horizon 2020 has concluded, its legacy lives on, influencing healthcare advancements with its extensive research funding. Horizon Europe takes up the mantle with a staggering budget of over €95 billion, dedicating more than €8 billion to tackle healthcare challenges head-on. These programs are the backbone of the EU’s mission to foster excellence in science and innovation, aiming for a healthier future for all its citizens.
Digital Health: A Leap Towards Efficiency and Accessibility
The rapid pivot to digital health solutions, spurred by the COVID-19 pandemic, has been nothing short of revolutionary. Initiatives such as Digital Health Europe and the “eHealth” Network are harmonising efforts across the continent, ensuring that digital advancements lead to tangible improvements in healthcare quality and efficiency.
These initiatives aim to improve healthcare by prioritising individual care, making health data sharing smooth, and promoting healthy lifestyles. Their goal is to create a healthcare system that works efficiently across all countries.
Preparedness and Equity: Addressing Today’s Urgencies and Tomorrow’s Needs
The establishment of the Health Emergency Preparedness Response Authority (HERA) marks a significant step in Europe’s commitment to being prepared for health crises. Alongside, the Pharmaceutical Strategy for Europe and the EU4Health Programme are bolstering the continent’s emergency response capabilities and addressing the wider spectrum of healthcare needs, from cancer prevention to promoting healthier lifestyles.
A critical aspect of these endeavors is bridging the health gap. The EU is keenly aware of the disparities in healthcare quality and accessibility across regions and is actively working to mitigate these through initiatives like the EU Regional Development Fund. These efforts are crucial in ensuring that the march towards innovation does not leave anyone behind, focusing on inclusivity and equality in healthcare access.
Legislation and Procurement: The Framework for Sustainable Healthcare
The journey towards a future-proof healthcare system in Europe is also navigated through significant legislative and procurement reforms. From the German Supply Chain Due Diligence Act to the Clinical Trial Regulation, the EU is setting new standards that emphasize responsibility, sustainability, and value-based procurement. These changes are instrumental in ensuring that the healthcare sector not only meets the current needs but does so in a way that is financially and environmentally sustainable.
At Vamstar, we recognise the complexities and the challenges of navigating the European healthcare landscape. Since our inception in 2019, we’ve harnessed the power of artificial intelligence to streamline the lifescience procurement process, championing value-based procurement to ensure that healthcare providers can access the best solutions at the best value. We are committed to supporting Europe’s vision of a resilient, innovative, and inclusive healthcare system for all.
Europe’s lifescience revolution is a testament to the power of unity, innovation, and foresight. Through collaborative efforts, strategic funding, and a commitment to inclusivity and sustainability, the EU is setting a global standard for what it means to build a healthcare system that is truly future-proof.
Other Materials
Book a 30 minutes meeting with us
Welcome to our scheduling page! Please choose an available date below to get started.
30 minutes meeting
We’ll email you the meeting link
6 minutes read
Navigating the Future of Pricing with AI: Pricing Co-Pilot

In the complex and fast-evolving landscape of global markets, the strategic importance of pricing can hardly be overstated. It’s the linchpin that not only affects revenue and margins but also determines market competitiveness.
This is where Artificial Intelligence (AI) steps in, revolutionising the way industries approach pricing strategies. In particular, the implementation of AI in tender and RFP (Request for Proposal) pricing across Italy, Spain, France, the Nordics, and other EU & ME markets has been nothing short of transformative.
The AI-Driven Pricing Revolution
AI technology has opened new avenues for analysing historical data, recognising patterns of wins and losses, and applying these insights to future tenders and RFPs. This analytical prowess has empowered businesses with predictions and scenarios rooted in real-life outcomes, leading to substantial revenue growth — ranging from 12% to 25% — and enhanced margins by 17% to 25% across diverse markets and assets.
Our Three-Phased Approach to Pricing
Our journey towards pricing is meticulously structured into three phases, each designed to build upon the insights and foundations laid in the preceding steps.
Phase 1: Data Discovery, Cleansing, and Enrichment
The first step in the process is to meticulously curate and enhance the dataset, ensuring its integrity and richness. This involves a thorough examination of the data to identify any inconsistencies, errors, or missing information that could potentially undermine the accuracy of the predictive models. Once these issues are detected, the data undergoes a rigorous cleansing process to correct the invalid entries and ensure the dataset’s overall quality.
However, the preparation phase goes beyond mere data cleaning. To truly unlock the potential of the predictive models, it is essential to enrich the dataset with valuable market insights. This enrichment process involves integrating relevant external data sources, such as industry trends, competitive intelligence, and regulatory information, to provide a more comprehensive and contextual understanding of the market dynamics.
By combining the internal data with these external insights, the dataset becomes a powerful asset that can drive more accurate and actionable predictions. This solid foundation of clean, enriched data sets the stage for the development of robust and reliable predictive models in the subsequent phases of the project.
Phase 2: Model Building
In this phase, the focus is on developing sophisticated predictive models that incorporate a vast array of variables. These models are designed to tackle complex challenges, such as forecasting prices at the molecular level and identifying the most likely winning bids for individual stock-keeping units (SKUs).
The algorithms take into account a wide range of factors that influence the pricing of drugs or medical products throughout their entire lifecycle, from initial launch to post-patent expiry scenarios. By considering the impact of various market dynamics, regulatory changes, and competitive landscapes, these models provide valuable insights into pricing strategies and help organisations navigate the complexities of the pharmaceutical and healthcare industries. The ultimate goal is to empower local teams with data-driven recommendations that optimise revenue, maximise profitability, and ensure sustainable growth in an increasingly competitive market.
Phase 2: Model Building
In this phase, the focus is on developing sophisticated predictive models that incorporate a vast array of variables. These models are designed to tackle complex challenges, such as forecasting prices at the molecular level and identifying the most likely winning bids for individual stock-keeping units (SKUs).
The algorithms take into account a wide range of factors that influence the pricing of drugs or medical products throughout their entire lifecycle, from initial launch to post-patent expiry scenarios. By considering the impact of various market dynamics, regulatory changes, and competitive landscapes, these models provide valuable insights into pricing strategies and help organisations navigate the complexities of the pharmaceutical and healthcare industries. The ultimate goal is to empower local teams with data-driven recommendations that optimise revenue, maximise profitability, and ensure sustainable growth in an increasingly competitive market.
The Vamstar Difference
The drive for greater commercial efficiency has become increasingly urgent against a backdrop of inflation, shortages, and the shift towards value-based healthcare. Vamstar distinguishes itself by leveraging AI to orchestrate, analyse, and provide intelligence on MedTech and Pharmaceutical data. This approach not only enhances market visibility but also optimises pricing strategies, thereby simplifying and automating commercial workflows to achieve sales excellence.
The Impact
Adopting AI in pricing does more than just improve financial metrics; it represents a paradigm shift in how businesses approach the market. By providing a granular view of demand and supply dynamics, and facilitating informed decision-making, AI technologies like those offered by Pricing Co-Pilot are setting new standards for efficiency and competitiveness in the healthcare sector.
Book A Demo Call Today
Unlock the future of pricing strategies with Vamstar’s AI Pricing Co-Pilot. Pre-register now to stay ahead with the latest innovations in healthcare pricing.
Transform your approach with data-driven insights and optimisation techniques designed for the healthcare and pharmaceutical industries.
Be the first to leverage AI-powered pricing strategies that drive growth and competitiveness. Click here to pre-register and lead the transformation in your sector.
Conclusion
The integration of AI into pricing strategies marks a significant leap forward for industries striving to navigate the complexities of modern markets. With its proven track record of enhancing revenues and margins, AI offers a promising path to not just survive but thrive in the competitive landscape. As we continue to explore and refine these technologies, the possibilities for innovation and improvement in pricing strategies are boundless.
Other Materials
Book a 30 minutes meeting with us
Welcome to our scheduling page! Please choose an available date below to get started.
30 minutes meeting
We’ll email you the meeting link
6 minutes read
NHS Supply Chain and the Future of NHS Procurement
Background:
The House of Commons Committee on Public Accounts released a report on March 20th, 2024, addressing the inefficiencies in the NHS Supply Chain procurement processes.
The report shed light on various challenges, including difficulties in achieving market share targets, oversight problems, lack of trust among stakeholders, and delayed transformation initiatives.
In this article, we explore the context of the report and propose recommendations for implementing an AI-based data orchestration platform to foster consistency, trust, and transparency in the procurement processes.
Introduction:
The NHS spends approximately £8 billion annually on medical equipment and consumables. NHS Supply Chain, established in 2018, aims to deliver savings and increase market share by aggregating spending power and reducing price variations.
However, challenges persist in persuading trusts to utilise the NHS Supply Chain, resulting in missed savings opportunities. This paper explores how AI-based data orchestration systems can address these challenges and improve NHS Supply Chain’s efficiency and patient outcomes.
Key Challenges:
- Low Trust Participation: NHS Supply Chain has failed to persuade trusts to use its services, resulting in only 57% market share against a target of 80% by 2023-24. This limits potential savings and efficiency gains.
- Weak Oversight and Support: NHSE has been weak in its oversight and support of NHS Supply Chain, failing to validate claimed savings and provide adequate financial support for modernization efforts.
- Lack of Trust Accountability: NHSE does not effectively challenge trusts to purchase more through the NHS Supply Chain, relying on trusts to analyse procurement data and change practices independently.
- Inconsistent Savings Reporting: NHS Supply Chain has used multiple methods to calculate and report savings, causing confusion and mistrust among trusts.
- Delayed Transformation Benefits: NHS Supply Chain’s transformation program, aimed at improving its business, is expected to run from 2022-30. Benefits will take several years to materialise due to capacity constraints and legacy system challenges.
- Balancing Cost and Quality: There are concerns that a focus on costs may impact product quality and patient outcomes. Clinicians need to be more involved in purchasing choices to ensure patient care is considered alongside value and cost.
To address these challenges and enhance the efficiency of the NHS Supply Chain, we propose the implementation of an AI-driven data orchestration technology and analytics to reduce risk to the NHS. This technology will ensure data consistency, build trust, and promote transparency in the procurement processes.
AI-based Data Orchestration Solution will support:
- Predictive Demand Forecasting: Implement AI algorithms to analyse historical procurement data, patient demographics, and clinical trends to accurately forecast demand for medical equipment and consumables. This will enable NHS Supply Chain to optimise inventory levels, reduce stockouts, and improve trust participation by ensuring product availability.
- Dynamic Pricing Optimisation: Develop an AI-powered pricing engine that continuously analyses market conditions, supplier contracts, and trust purchasing patterns to offer competitive and transparent prices. This builds trust and confidence, and encourages increased utilisation of the NHS Supply Chain.
- Intelligent Procurement Analytics: Deploy AI-driven analytics to identify purchasing patterns, price variations, and potential savings opportunities across trusts. Provide actionable insights to NHSE and trusts, enabling data-driven challenges and accountability for utilising NHS Supply Chain.
- Unified Savings Reporting: Establish a standardised, AI-powered savings calculation methodology that integrates data from NHS Supply Chain, trusts, and suppliers. Ensure consistency, transparency, and trust in reported savings across all stakeholders.
- AI-assisted Transformation Management: Leverage AI project management tools to optimise resource allocation, identify critical paths, and monitor progress of NHS Supply Chain’s transformation program. Use predictive analytics to anticipate and mitigate risks, ensuring timely delivery of modernisation benefits.
- Value-based Procurement: Implement an AI framework that incorporates clinical outcomes, patient satisfaction, and long-term cost savings into procurement decisions. Engage clinicians in defining value metrics and utilise AI to analyse real-world evidence, ensuring a balance between cost and quality.
Conclusion:
Implementing AI-based data orchestration systems can significantly enhance NHS Supply Chain’s efficiency, savings, and patient outcomes. By leveraging predictive analytics, dynamic pricing, intelligent procurement insights, unified savings reporting, AI-assisted transformation management, and value-based procurement, NHS Supply Chain can overcome existing challenges and drive trust participation. Collaboration among NHS Supply Chain, NHSE, trusts, and clinicians is crucial to realise the full potential of these AI solutions and ensure a sustainable, patient-centric procurement process.
Find Out More About Data Orchestration
Register to discover how AI-driven data orchestration can unlock unprecedented efficiency, savings, and improved patient outcomes within the NHS and other European National Healthcare Services.
Register
Please provide your details and we’ll contact you
Other Materials
6 minutes read
Leveraging AI & Data to Create a Compelling Go-To-Market “GTM” Strategy for a MedTech Product
Unlocking Insights from Tender or RFP Data
Tender data contains a wealth of information that can inform various aspects of a MedTech company’s GTM strategy. Some of the key insights that can be gleaned from tender documents include:
1. Product Availability: Tender notices and documents often specify the types of products required, along with their technical specifications and desired features. This information can help MedTech companies understand the range of products available in the market and identify gaps or opportunities for innovation.
2. Supplier Landscape: Tender award notifications reveal the successful bidders, providing insights into the competitive landscape. By analysing this information, MedTech companies can identify their key competitors, assess their market share, track price movements, and monitor their activities.
3. Pricing and Discounts: In many markets, tender documents disclose the prices and discounts offered by suppliers. This transparency allows MedTech companies to benchmark their pricing strategies, understand the price sensitivity of buyers, and identify opportunities for competitive positioning.
4. Buyer Preferences: Tender documents often outline the evaluation criteria used by buyers to assess bids. These criteria may include factors such as product quality, after-sales support, training, and sustainability. By understanding buyer preferences, MedTech companies can tailor their offerings and value propositions to better meet customer needs.
5. Market Conditions: Tender data can provide insights into market-specific conditions, such as regulatory requirements, local content requirements, and payment terms. This information is crucial for MedTech companies looking to enter new markets or expand their presence in existing ones.
6. Non-Price Factors: In addition to price, tender documents may specify non-price factors that influence the award decision, such as technical capabilities, clinical evidence, and supplier reputation. MedTech companies can use this information to differentiate themselves and highlight their unique strengths.
The Tender or the RFP Landscape
Tendering is a highly regulated process that aims to ensure fair competition, transparency, and value for money in public procurement. Governments and healthcare institutions issue tenders to invite suppliers to submit bids for the provision of goods or services. The tender process typically involves several stages:
1. Tender Announcement: The buyer publishes a notice outlining their requirements, specifications, and timeline for the procurement process.
2. Tender Documents: Interested suppliers can obtain detailed tender documents that provide further information on the scope of work, evaluation criteria, and submission requirements.
3. Bid Submission: Suppliers prepare and submit their bids, which include technical and commercial proposals, as well as supporting documentation.
4. Bid Evaluation: The buyer evaluates the submitted bids based on predefined criteria, which may include price, quality, technical capabilities, and other factors.
5. Award Notification: The buyer announces the winning bidder and provides feedback to unsuccessful participants.
6. Award Documents: The buyer and the winning supplier sign a contract outlining the terms and conditions of the agreement.
Throughout this process, a significant amount of information is generated and made available to the public or semi-public, albeit for a limited time. This information can provide invaluable insights into the competitive landscape, market trends, and customer needs.
Introduction
In the highly competitive and regulated world of medical technology “MedTech”, crafting a compelling GTM strategy is crucial for success. While the United States market relies heavily on direct sales and GPO-level negotiations, the majority of the global market operates through the tender channel (also referred to as Request For Proposals “RFPs”).
Tender notices (pre-tender and post-tender), documents, award notifications, and award documents contain a wealth of valuable information that can inform and shape a MedTech company’s GTM strategy. By systematically analysing this data, sales, marketing, market access, commercial, and pricing teams can gain insights into market dynamics, competitor activities, pricing trends, and buyer preferences. However, the process of extracting, organising, and integrating this information can be challenging due to its semi-structured and unstructured nature, limited public availability, and the need to merge it with internal datasets.
This article will explore the importance of tender data in developing a robust GTM strategy for MedTech products and provide guidance on how to effectively utilise this information to drive success in institutional markets.
Challenges in Extracting and Utilising Tender Data
While tender/ RFP documents offer immense value, extracting and utilising this information can be challenging for several reasons:
1. Semi-Structured and Unstructured Data: Tender documents are often in the form of PDFs, Word documents, or HTML pages, which contain a mix of structured and unstructured data. Extracting relevant information from these sources requires advanced data parsing and natural language processing techniques.
2. Limited Public Availability: Tender notices and documents are typically available in the public domain for a limited period, often ranging from a few weeks to a month. This short window poses challenges for teams trying to collect and analyse the data manually.
3. Data Integration: To derive maximum value from tender data, it needs to be integrated with internal sales, pricing, and market intelligence datasets. This integration allows for the identification of patterns, trends, and correlations across markets and product segments. However, merging disparate data sources can be complex and time-consuming.
4. Language and Localisation: Tenders are often published in local languages and may use country-specific terminology and formats. MedTech companies operating in multiple geographies need to overcome language barriers and ensure consistent data interpretation across markets.
Leveraging Technology for Tender Data Analysis
To address the challenges of extracting and utilising tender data, MedTech companies can leverage advanced technologies such as:
1. Web Scraping: Automated web scraping tools can systematically extract tender data from online portals, websites, and databases. These tools can handle large volumes of data and navigate complex website structures to capture relevant information.
2. Optical Character Recognition “OCR”: OCR technology can convert scanned tender documents and images into machine-readable text, enabling the extraction of structured data from unstructured sources.
3. Natural Language Processing “NLP”: NLP algorithms can analyse the text in tender documents to identify key information such as product specifications, evaluation criteria, and price points. NLP can also help in language translation and localisation.
4. Data Orchestration Platforms: Specialised data integration platforms can streamline the process of merging tender data with internal datasets. These platforms can handle data cleansing, standardisation, and harmonisation, ensuring a consistent and reliable data foundation for analysis.
Applying Tender Insights to GTM Strategies
Once the tender data has been extracted and integrated, MedTech companies can use the insights to inform and optimise their GTM strategies across the four key dimensions of pricing, positioning, place, and promotion.
1. Pricing: Tender data provides visibility into the pricing strategies of competitors and the price sensitivity of buyers. MedTech companies can use net price information to develop competitive pricing models, offer targeted discounts, and negotiate more effectively with procurement teams.
2. Positioning: By analysing tender evaluation criteria and buyer preferences, MedTech companies can refine their product positioning and value propositions. This may involve highlighting unique product features, emphasising clinical benefits, or showcasing after-sales support capabilities to differentiate from competitors.
3. Place: Tender data can reveal market-specific requirements and conditions that impact the distribution and logistics of MedTech products. Companies can use this information to optimise their supply chain, establish local partnerships, and ensure compliance with regulations.
4. Promotion: Tender notices and award notifications provide insights into the decision-makers and influencers involved in the procurement process. MedTech companies can leverage this information to tailor their promotional activities, such as targeted marketing campaigns, key opinion leader engagement, and educational initiatives.
In addition to the four Ps, tender data can also shed light on the regulatory landscape and its impact on institutional markets. By monitoring tender requirements and specifications, MedTech companies can stay abreast of evolving regulations, standards, and certifications. This knowledge can help companies proactively adapt their products and processes to meet changing market demands.
Contact Us Today to Elevate Your MedTech GTM Strategy
Unlock the full potential of your MedTech product with data-driven insights from tender documents. Don’t let the complexities of market entry, pricing, or competitor analysis hold you back.
Use the form below to get in touch with our team of experts who specialise in leveraging AI and sophisticated data analysis to refine your Go-To-Market strategy.
We are here to help you navigate the tender process efficiently and enhance your market success. Contact us now to schedule a consultation and see how we can assist you in achieving a sustainable competitive advantage.

Contact Us
Please provide your details and we’ll contact you shortly.
Conclusion
Tender data represents a goldmine of insights for MedTech companies looking to develop effective GTM strategies in institutional markets. By systematically analysing tender notices, documents, award notifications, and award documents, companies can gain a deep understanding of market dynamics, competitor activities, pricing trends, and buyer preferences.
However, extracting and utilising tender data poses significant challenges due to its semi-structured and unstructured nature, limited public availability, and the need for data integration. To overcome these challenges, MedTech companies must leverage advanced technologies such as web scraping, OCR, NLP, and data integration platforms.
By applying the insights derived from tender data to the four key dimensions of pricing, positioning, place, and promotion, MedTech companies can create compelling GTM strategies that resonate with customers, differentiate from competitors, and drive market success. Additionally, monitoring the regulatory landscape through tender data can help companies stay compliant and adapt to changing market requirements.
In conclusion, MedTech companies that invest in the systematic analysis of tender data and integrate the insights into their GTM strategies will be well-positioned to navigate the complexities of institutional markets, drive growth, and achieve a sustainable competitive advantage.
Vamstar
Vamstar can play a crucial role in enhancing the effectiveness of a MedTech company’s GTM strategy by addressing the challenges associated with extracting, analysing, and utilising tender data at scale and asynchronously. By leveraging advanced technologies such as web scraping, OCR, NLP, Machine Learning and data integration, Vamstar can automate the process of collecting and organising tender information from various sources, including all internal datasets from CRM, Price/Revenue Management Systems or ERP systems. This not only saves time and resources but also ensures a comprehensive and up-to-date dataset that can be easily accessed and analysed by different teams within the organisation.
Moreover, Vamstar’s platform can provide a centralised repository for tender data, enabling seamless integration with internal sales, pricing, and market intelligence systems. This integration allows for the identification of patterns, trends, and correlations across markets and product segments, empowering MedTech companies to make data-driven decisions and adapt their GTM strategies in real-time. By leveraging the insights derived from Vamstar’s platform, MedTech companies can optimise their pricing models, refine product positioning, streamline distribution channels, and tailor promotional activities to better meet the needs of their target customers and stay ahead of the competition.
Other Materials
Book a 30 minutes meeting with us
Welcome to our scheduling page! Please choose an available date below to get started.
30 minutes meeting
We’ll email you the meeting link
6 minutes read
Using generative AI, knowledge graphs and natural language processing in MedTech product and code matching A
Medical supplies include a wide range of products, from surgical instruments to bandages, that are used in healthcare facilities. Properly classifying and assigning codes to these medical supplies is critical for sourcing, tracking, billing, ordering, inventory management, and patient safety.
Key challenges include the high cost of healthcare supply-chain transactions (up to 4x higher compared to other industries), significant waste incurred e.g. $5Bn worth of COVID-19 PPE deemed ‘unusable’. In addition globally, 10% to 34% of health care spendings of OCED countries were wasted on inappropriate care.
We have broken down the challenge into code-to-code, code-to-product, product-to-product, product-to-evidence, and product-to-opportunity. More specifically
- Code-to-code: suppose you have one code such as CPV and you want to match this to your own internal code or other codes such as UNSPSC.
- Code-to-product: imagine as a buyer you onboard a new supplier with 30,000 items or items that you need to assign categories to. As a supplier I need to filter or assign buyer code to my products.
- Product-to-product: as a buyer I have lots of products in my basket of goods, of different brands or suppliers, how can you compare the products to find similar ones? As a supplier how can I understand the market landscape.
- Product-to-evidence: now as a buyer you want to screen the clinical evidence of many items, how do you do this at the moment? as a supplier I want to see what my competitors are doing
- Product-to-opportunity: as a buyer I want to see the supplier products, or as a supplier I want to see the opportunities I can participate in or my competitors are doing
Here is a selection of complexities involved in classification:
- Complexity, Diversity, and Standardisation Issues: The sheer variety of medical supplies, each with its unique specifications, makes classification intricate. Differentiating between products with minor variations can be challenging. There may be a lack of standardised naming conventions and categorisations for medical supplies, especially across different manufacturers or countries. Different vendors might use different names, codes, or specifications for similar products, making it challenging to maintain a standardised classification.
- Continuous Evolution of Products: As medical technology advances, new products are constantly introduced to the market. Keeping classification systems up-to-date becomes an ongoing task.
Overlapping Categories: Some medical supplies can fit into multiple categories, leading to confusion about proper classification. - Human Errors, Scale and Skill: Mistakes can occur in manual entry or categorisation processes, leading to misclassifications. Staff needs to be trained to understand and use the classification system correctly, and this training has to be updated as the system evolves.
- Regulatory and Compliance Requirements: Different regions or countries might have different regulations concerning medical supplies, which can affect how they need to be classified or coded. In multinational settings, translation and localisation can introduce further complexity to classification.
- Interoperability and Integration: Different systems within a healthcare facility, like billing, electronic health records, and inventory management, need to communicate seamlessly. Discrepancies in code classifications can lead to issues in this integration. Legacy systems in hospitals or facilities might still rely on outdated classification systems, leading to discrepancies when integrating with newer systems or vendors. Classification systems need to be compatible with inventory management systems to ensure seamless tracking of medical supplies usage and restocking needs.
Addressing these challenges requires a combination of technology, training, and careful planning. Solutions might include investing in modern inventory management systems, providing ongoing staff training, collaborating with vendors for standardisation, and regularly reviewing and updating classification systems. Doing this manually globally for all healthcare products and services is not possible and this needs an automated tracking and monitoring solution, luckily with the emergence of big data, generative AI, and graph analytics this is possible.
While ChatGPT-4 might be good at summarising text, our data scientists have found that using it for code-to-code matching such as GMDN to UNSPSC leads to incorrect classification code being assigned even after careful data preparation. These inaccurate responses are a process known as hallucination and has widely been discussed in the press as a major issue on trust and quality. When you match product-to-code things can also quickly go out of hand due to the noise.
Vamstar has deep experience in cutting edge data science and artificial intelligence in the Healthcare and MedTech sectors. We have been awarded leading Innovate UK Research and AI Venture funding, and have an active collaboration with leading Universities.
We have developed ground-breaking solutions in the form of a platform driven by AI-technologies, to allow the standardisation and automated cataloging of healthcare products/services, enriched with information harvested from heterogeneous sources to enhance procurement decision making.
We have collected, extracted using NLP, normalised, and enriched the largest public tendering datasets in the world from buyers such as hospitals, clinics, and universities. We for example know the live tender opportunities for MedTech products, the awarded suppliers, and purchasing patterns.
In parallel we have collected, normalised, and enriched products and items from manufacturer, supplier and distributor catalogues using custom natural language processing and generative AI. Giving us a full understanding of the supply-side portfolios and offerings.
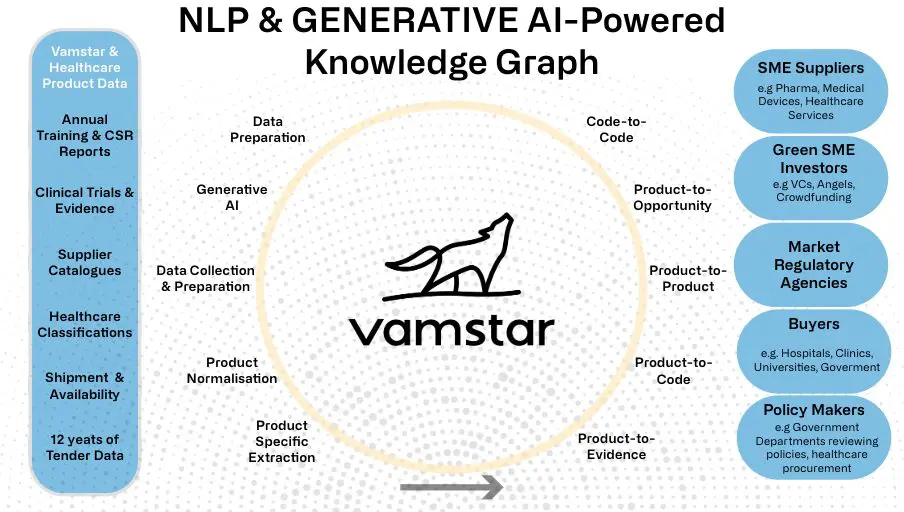
We combined these two massive healthcare and MedTech specific datasets, and created the largest knowledge graph that allows us to perform graph matching of products and codes at hyperscale never seen before, using low level product features, and specifications in healthcare across languages.
Creating the world’s largest healthcare and life sciences knowledge graph – interconnecting all healthcare buyers, suppliers, products, services, and medical devices across all countries is a complex task that needs unique skill sets, and a lot of normalised and enriched data. The underlying graph was created using complex natural language processing (NLP), Generative AI, and Machine Learning models including the use of GPU instances for training our custom deep learning NLP models. In collaboration with the University of Sheffield, global leaders in NLP, we scaled out both in terms of data we process and in terms of the nodes and relationships represented in the network graph.
We have layered three state-of-the-art technologies of Generative AI, Natural Language Processing, and Knowledge Graphs together in perfect harmony to auto-match all of your code and product assignment and classifications. This is orchestrated using a complex series of proprietary big data and machine learning pipelines that easily scale in the Cloud.
We have the unique ability to perform highly scalable and accurate:
- Code-to-code: Auto matching CPV, UNSPC, GMDN, GUDID, UDI, eClass and internal code/catalogue/taxonomy matching.
- Code-to-product: Assign CPV, UNSPC, eClass or internal code/catalogue/taxonomy to products.
- Product-to-product: compare products with other products, and provide a matching score.
- Product-to-evidence: summarise the clinical evidence for a product or group of products.
- Product-to-opportunity: we have the ability to match a tender or private opportunity to a supplier products.
Vamstar is the leading AI-powered B2B Healthcare and Lifesciences Exchange platform, aggregating and analysing over $750 billion in spending across 86,000 buyers from public and private healthcare product and service sources in 100+ countries. Vamstar’s cloud-based supply chain technology connects both buyers and suppliers to automate key business processes, translating data and outcome-based analytics into meaningful action for the healthcare ecosystem to move fast, operate efficiently and achieve great synergies, enabling better patient care and maximizing industry savings for its clients.
We use Big Data and Machine learning to enable smart sourcing, faster tendering, simplified contracting, real-time opportunities, embedded intelligence, and related services, including the web-based trading of healthcare products between hospitals, clinics, laboratories, and suppliers in the market.
By seeing the big picture and all the connections, Vamstar provides healthcare stakeholders with valuable market insights and perspectives. Vamstar partners with leaders in the industry, academia, and government in 100+ countries to apply higher-level thinking to daily tasks and strategic issues. They offer their customers solutions to make them more efficient and help them make data-driven informed decisions to secure their future.
Other Materials
Book a 30 minutes meeting with us
Welcome to our scheduling page! Please choose an available date below to get started.
30 minutes meeting
We’ll email you the meeting link