Category: MedTech
4 minutes read
Tuning into the Ambiguity of VBP and ESG: How Vamstar is Simplifying Complexity
In the rapidly evolving landscape of healthcare procurement and sustainability, Value-Based Procurement (VBP) and Environmental, Social, and Governance (ESG) initiatives stand as two interconnected yet ambiguous pillars of transformation.
VBP focuses on shifting the healthcare procurement paradigm from cost-driven to value-driven approaches, emphasising patient outcomes, efficiency, and quality. ESG, on the other hand, encapsulates the integration of environmental, social, and governance considerations into organisational strategies, ensuring long-term sustainability and ethical operations. Organisations are increasingly required to navigate the fine balance between cost efficiency, sustainability, and patient-centric outcomes.
This complexity arises from fragmented frameworks and the absence of standardised benchmarks, leaving organisations to interpret and adapt broad principles to their unique procurement strategies. For instance, balancing short-term cost savings with long-term environmental impacts or aligning patient-centric care models with financial sustainability demands a multidimensional approach that bridges these gaps. Vamstar’s AI-driven capabilities are positioned at the heart of this challenge, offering clarity amidst the complexity.
Understanding the Ambiguity
Both VBP and ESG are rooted in noble goals: delivering better patient outcomes and ensuring long-term sustainability. Yet, they come with inherent ambiguities:
- VBP’s Evolving Definition: While VBP shifts the focus from price to value, what constitutes “value” often varies between stakeholders—healthcare providers, suppliers, and patients. The lack of standardised metrics creates challenges in defining success. Stakeholders might use metrics such as patient outcomes, cost-efficiency benchmarks, or environmental impact scores, yet these vary widely. Establishing universal guidelines or collaborative frameworks could provide a pathway toward clearer and more actionable definitions of value.
- ESG’s Operational Disconnect: Despite its growing importance, ESG often struggles to integrate seamlessly into procurement strategies. Companies face hurdles in aligning operational processes with high-level sustainability goals, particularly when tracking data or demonstrating tangible impact.
This dual ambiguity leaves healthcare organisations grappling with questions: How do we measure success in VBP? How do ESG principles translate into procurement practices? And most importantly, how can they work together?
Vamstar: Illuminating the Path Forward
At Vamstar, we recognise that ambiguity in VBP and ESG is not a roadblock—it’s an opportunity for innovation. Our AI solutions, including ValueGPT, are designed to streamline the intersection of these frameworks, empowering healthcare organisations to make data-driven decisions that deliver measurable outcomes.
- Data Harmonisation: By leveraging advanced AI, we consolidate and classify diverse datasets, transforming fragmented information into actionable insights for both VBP and ESG strategies.
- Evidence Mapping: Vamstar’s tools map clinical and sustainability evidence, providing a clear view of how procurement choices impact patient outcomes and ESG compliance.
- Policy Tracking: With real-time analysis of global and regional regulations, we help organisations align their procurement strategies with evolving standards in sustainability and value measurement.
Creating a Unified Framework
The convergence of VBP and ESG is not just a challenge; it’s a necessity for the future of healthcare. At Vamstar, we advocate for an integrated approach where procurement decisions are informed by both value and sustainability metrics. Our solutions enable stakeholders to:
- Define Clear Metrics: Establish robust, transparent criteria for evaluating both value and sustainability in procurement processes.
- Enhance Collaboration: Foster stronger partnerships between suppliers, providers, and policymakers through shared data and unified goals.
- Drive Accountability: Ensure every decision aligns with both patient outcomes and long-term sustainability objectives.
The Road Ahead
Healthcare organisations must evolve from ambiguity to action. The future demands solutions that not only address today’s complexities but also anticipate tomorrow’s challenges. At Vamstar, we are committed to bridging the gap between VBP and ESG, transforming these frameworks into actionable, impactful strategies that drive measurable change.
Our approach is not just about simplifying processes but creating a paradigm shift where sustainability and value are seamlessly integrated. By leveraging cutting-edge AI solutions like ValueGPT, we help organisations align their procurement strategies with global standards while fostering innovation and accountability. This ensures not only compliance but also meaningful contributions to the healthcare ecosystem.
The journey may be complex, but with the right tools, collaborative partnerships, and a forward-thinking mindset, the ambiguity of VBP and ESG becomes an opportunity to lead. Vamstar is here to guide that journey, enabling healthcare organisations to achieve sustainable, value-driven growth that benefits patients, stakeholders, and the planet alike.
Explore what AI can do for your organisation
Discover how we can empower your business with innovative solutions and drive growth.
Contact Us Today
Please provide your details and we’ll contact you.
6 minutes read
COP29: The Integration of Health into Climate Goals and Its Implications for MedTech and Pharma

The recent COP29 summit marked a pivotal moment in the global effort to combat climate change, with a growing emphasis on the intersection of health and climate policies. For the first time, countries were urged to integrate health considerations into their Nationally Determined Contributions (NDCs)—commitments under the Paris Agreement outlining strategies to reduce emissions and adapt to climate change. This development carries profound implications for the MedTech and Pharmaceutical industries, which stand at the nexus of healthcare innovation and sustainability.
Health in NDCs: A New Era of Climate Policy
Traditionally, NDCs have focused on sectors like energy, transportation, and industry, leaving health concerns on the periphery. However, COP29 underscored the undeniable links between climate change and public health. Rising temperatures, worsening air quality, and the spread of vector-borne diseases such as malaria and dengue highlight the urgent need for climate-resilient healthcare systems.
By integrating health into their climate commitments, countries aim to align public health and environmental objectives. This shift involves recognising the dual benefits of climate action—such as reducing air pollution to lower emissions while improving health outcomes—and embedding these into national strategies.
Impacts on MedTech and Pharmaceutical Industries
- Regulatory Shifts - Governments may introduce new regulations requiring MedTech and pharmaceutical companies to adopt sustainable practices. This could include mandates to reduce carbon emissions from manufacturing, transition to renewable energy, or incorporate eco-friendly packaging for medical devices and drugs.
- Resilient Healthcare Infrastructure - Climate-smart healthcare systems will demand innovative MedTech solutions. For instance, energy-efficient medical devices, portable diagnostic tools for disaster zones, and tele-health platforms could see increased demand as countries prioritise climate resilience.
- Funding for Green Innovation - Nations aligning health and climate goals could create incentives for companies to invest in green technologies. This might include funding for research into sustainable pharmaceuticals or the development of MedTech solutions that address climate-induced health challenges.
- Transparency and Accountability - With health embedded in NDCs, governments might enforce stricter reporting requirements for the environmental impacts of pharmaceutical and MedTech operations. Companies would need to link sustainability metrics—such as carbon footprints—to public health outcomes.
- Emerging Market Opportunities - The shift also opens new markets for products addressing climate-related health issues. For example, diagnostic tools for diseases exacerbated by changing climates, such as respiratory conditions linked to pollution, or heat-stress monitoring devices for vulnerable populations.
A Call to Action for MedTech and Pharma
- Adopt Climate-Smart Manufacturing: Transitioning to renewable energy sources and improving energy efficiency across facilities can align companies with stricter regulatory expectations.
- Innovate for Resilience: Developing devices and drugs tailored to climate-driven health challenges will be critical. For example, portable solutions for diagnostics in disaster-stricken areas or medications targeting emerging diseases can position companies as leaders in this evolving space.
- Collaborate with Governments and NGOs: Partnerships to develop and deploy climate-resilient healthcare solutions can drive widespread impact, ensuring equitable access to care in vulnerable regions.
The Road Ahead
The integration of health into Nationally Determined Contributions (NDCs) unveiled at COP29 marks a critical evolution in the global response to climate change. For the MedTech and pharmaceutical industries, this alignment is a clear signal to innovate and adapt. Embracing sustainability, operational excellence, and data-driven strategies will be essential to meet regulatory demands and capitalise on emerging opportunities in climate-smart healthcare.
At the forefront of this transformation, tools like Vamstar’s Value AI are poised to play a pivotal role. By leveraging advanced AI capabilities, MedTech and Pharma companies can efficiently map, track, and analyse evidence bases and policies to stay ahead of regulatory trends, optimise market access, and drive sustainability efforts. As the healthcare landscape evolves, solutions like Value AI empower organisations to seamlessly integrate sustainability into their strategic frameworks, ensuring they not only survive but thrive in this new era of health and climate convergence.
This moment demands bold action, and with the right tools and vision, MedTech and Pharma companies have the opportunity to lead the way toward a healthier, more sustainable future.
Discover Our Solutions
Discover how we can empower your business with Agentic AI solutions and drive growth.
Get in touch with us
Please provide your details and we’ll contact you
Other articles
9 minutes read
The Evolution of AI in Medical Devices: Regulatory Challenges and Future Directions
Artificial intelligence (AI) and machine learning (ML) are reshaping the MedTech landscape, driving advancements in diagnostic precision, personalised treatment, and operational efficiency. At the Advamed MedTech conference, industry leaders and regulatory representatives from Canada and the U.S. explored the integration of AI into medical devices, spotlighting its transformative potential and the regulatory frameworks needed to harness it responsibly.
As of August 2024, nearly 1,000 AI/ML-enabled medical devices have received regulatory approval. This milestone underscores AI’s growing prominence but also highlights a pressing need to address the unique challenges posed by adaptive, self-learning technologies. Ensuring safety, efficacy, and ethical use in a rapidly evolving field demands bold, forward-thinking regulatory strategies.

The AI Revolution in Medical Devices
AI in medical devices dates back to the 1990s, with early applications in imaging that relied on locked, static algorithms. Today, adaptive AI models dominate, capable of evolving with new data and contexts. These dynamic capabilities unlock revolutionary potential—personalised care, faster diagnostics, and smarter healthcare systems—but also present regulatory complexities far beyond traditional device oversight.
To keep pace, the MedTech industry and regulators are rethinking frameworks that historically relied on static product definitions and pre-market approvals. The result is a growing emphasis on continuous monitoring, adaptive oversight, and cross-sector collaboration.
Leading Regulatory Efforts: Health Canada and FDA
Regulatory agencies are stepping up to meet the demands of AI-driven innovation:
- Health Canada’s Digital Health Division: Established in 2018, this team oversees high-risk AI medical devices, focusing on cybersecurity, software, and adaptive learning technologies. It is instrumental in setting Canada-specific performance benchmarks and lifecycle guidelines.
- FDA’s Digital Health Center of Excellence: Pioneering frameworks for AI/ML in healthcare, the FDA is evolving its regulatory philosophy to balance rapid innovation with uncompromising safety standards.
The Key Regulatory Challenges
- Performance Degradation
Adaptive AI models can “drift,” where performance declines over time as data environments change. Regulators are pushing for real-time monitoring frameworks to ensure safety and efficacy throughout a device’s lifecycle. - Transparency and Explainability
The complexity of AI models often creates a “black box” effect, making it difficult to understand how decisions are made. Regulators are driving initiatives to improve transparency—enabling stakeholders to trust AI systems without necessarily understanding their full complexity. - Post-Market Surveillance
AI’s ability to evolve post-deployment necessitates a shift in regulatory focus from pre-market evaluations to robust, ongoing performance monitoring. Agencies are piloting adaptive models for oversight to align with AI’s continuous development. - Evolving Regulatory Frameworks
Decades-old regulatory structures, designed for static devices, are ill-suited for AI’s dynamic nature. Agencies like the FDA and Health Canada are redefining what constitutes a medical device, establishing iterative approval processes, and exploring pathways for rapid updates. - Cross-Site Deployment Challenges
AI models trained in one environment may underperform in different settings. Regulators and manufacturers are collaborating on protocols for local adaptation and validation to ensure consistent performance across diverse clinical contexts. - Healthcare Workforce Pressures
AI is increasingly viewed as a solution to alleviate workforce shortages. Regulators are balancing the need for AI deployment speed with safeguards to ensure human oversight, ethical integration, and clinician training. - Data Silos
Fragmented healthcare datasets hinder AI model development. Regulatory agencies are working to break down silos through frameworks for federated learning, synthetic data generation, and secure, privacy-compliant data sharing.
Regulatory Advances and Innovations
Health Canada’s Strategic Initiatives
- Machine Learning Guidance: A forthcoming guidance document will set clear lifecycle standards for AI devices, focusing on adaptability and continuous improvement.
- License Conditions: Tailored conditions ensure devices meet performance benchmarks even after deployment.
- Collaborative Stakeholder Engagement: Partnerships across industry, academia, and healthcare inform Canada’s regulatory priorities.
- Scientific Advisory Committee: This expert-led group guides digital health strategies, emphasizing AI policy development.
- Predetermined Change Control Plans (PCP): These plans enable manufacturers to implement pre-approved updates, reducing regulatory bottlenecks.
FDA’s Forward-Thinking Framework
- Standards Development: Aiming for universal benchmarks, the FDA is crafting evaluation standards tailored to AI technologies.
- Risk Management Protocols: Comprehensive quality assurance systems monitor AI lifecycle risks and mitigate evolving threats.
- Nuanced Evaluation: By distinguishing between predictive and generative AI, the FDA tailors regulatory reviews to the specific characteristics of each technology.
- Proactive Education: Outreach efforts are equipping stakeholders—from legislators to clinicians—with the knowledge to navigate AI’s integration.
Global Harmonisation
Initiatives such as the International Medical Device Regulators Forum (IMDRF) and joint publications from Health Canada, the FDA, and the UK’s MHRA aim to standardise global AI regulatory approaches, fostering consistency across jurisdictions.
Emerging Concepts Shaping Regulation
- Transparency vs. Explainability: Regulators are prioritising transparent communication of AI capabilities while recognising that full explainability may not be feasible for all models.
- Human-AI Collaboration: Frameworks increasingly consider AI as an augmentation tool, emphasising its integration into clinician workflows rather than standalone decision-making.
- Lifecycle Management: Adaptive oversight ensures safety across the entire AI device lifecycle, from development to decommissioning.
- Bias Mitigation: AI must demonstrate equity in outcomes across diverse patient populations, driving stricter subgroup analyses and bias identification protocols.
- Benefit-Risk Evolution: Regulatory assessments now weigh long-term, systemic benefits, even in cases where traditional statistical backing may be limited.
Future Directions
- Defining Medical Devices in an AI Era
AI blurs the line between software and hardware, prompting regulators to rethink device classifications and approval categories. - Accelerating Regulatory Timelines
Rolling submissions and phased approvals are being explored to keep pace with AI advancements without compromising safety. - Focus on Underserved Populations
Targeted incentives are encouraging AI development for paediatric care and underrepresented demographics, addressing equity gaps in healthcare. - Standardising Transparency
Frameworks like model cards and data cards will become essential tools for communicating AI limitations and capabilities. - System-Wide AI Integration
AI’s potential extends beyond devices to addressing workforce shortages and cost challenges. Regulators are exploring frameworks that consider AI’s broader systemic impact.
Conclusion
The integration of AI into medical devices represents one of the most significant paradigm shifts in modern healthcare. While the benefits—improved accuracy, personalised care, and operational efficiency—are immense, these advancements also demand regulatory frameworks that are as dynamic and adaptable as the technologies themselves.
By embracing lifecycle management, fostering transparency, and prioritising cross-stakeholder collaboration, regulators can strike the delicate balance between fostering innovation and safeguarding patient safety. As AI reshapes the MedTech landscape, its responsible integration will define the next era of healthcare, delivering transformative solutions to global challenges. The future is here, and it’s adaptive, intelligent, and deeply collaborative.
Discover Our Solutions
Discover how we can empower your business with Agentic AI solutions and drive growth.
Get in touch with us
Please provide your details and we’ll contact you
Other articles
7 minutes read
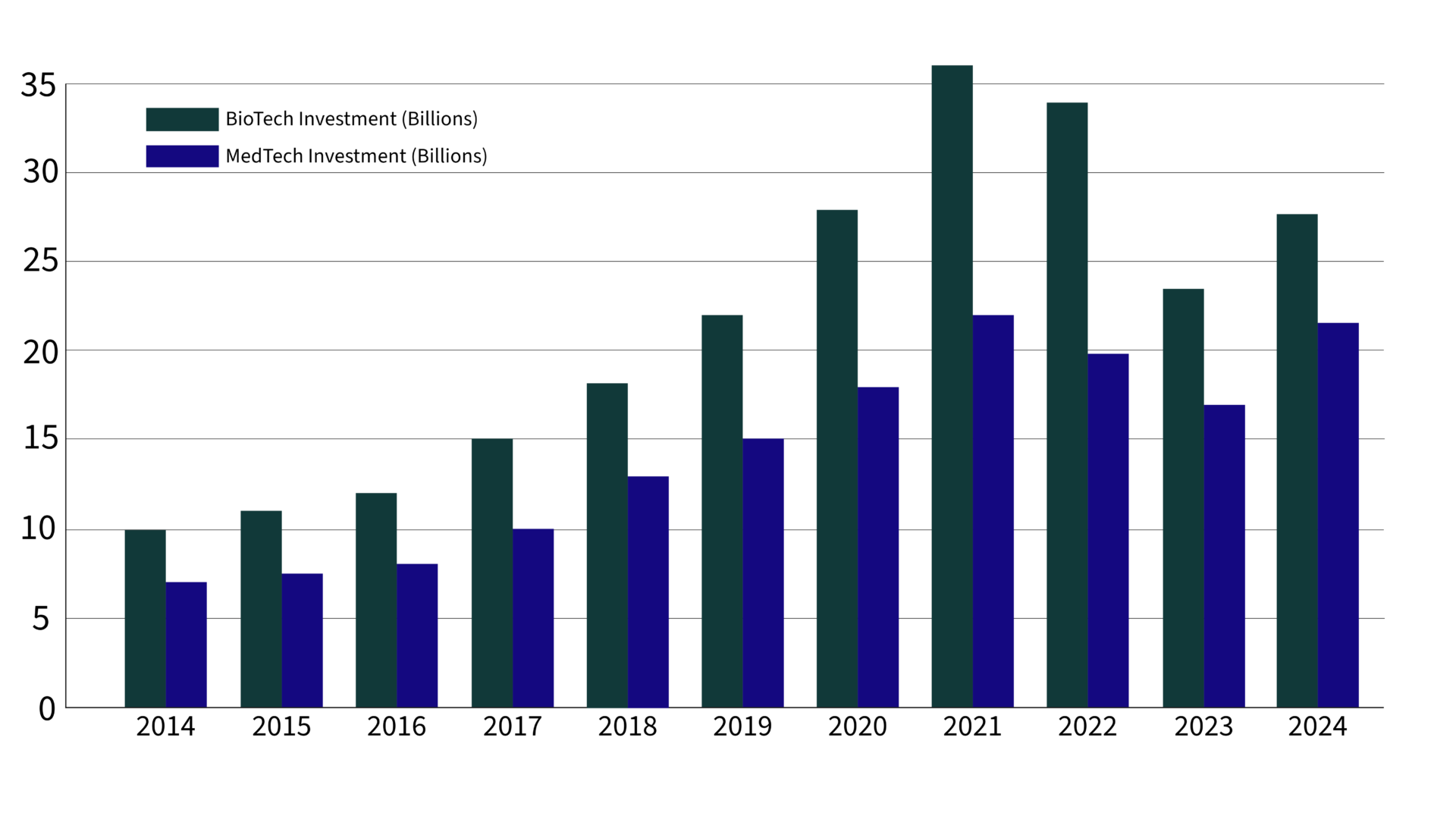
Biopharma and MedTech’s 2024 Investment Resurgence

After two years of cautious investing, venture capital is making a powerful comeback in biopharma and MedTech, signalling a reinvigorated confidence in the potential for healthcare innovation.
According to recent data from JPMorgan’s Q3 2024 report, venture investments in biopharma are poised to hit $27.7 billion this year, while MedTech is projected to see a 30% increase over 2023’s funding, reaching an impressive $21.5 billion.
This influx reflects strategic shifts towards fewer, larger investments and a renewed focus on high-impact, scalable technologies that promise to reshape patient care.
Register to continue reading.
4 minutes read
Dexcom Launches Stelo: A Game-Changer in the Wearables Market

Dexcom has taken a bold step in the wearables market with the launch of Stelo, its first over-the-counter (OTC) continuous glucose monitor (CGM). This device, specifically designed for adults with Type 2 diabetes who do not use insulin and those with pre-diabetes, is set to make advanced glucose monitoring more accessible and affordable than ever before.
The Significance of Stelo in the CGM Market
Stelo represents a significant shift in the CGM landscape, traditionally dominated by prescription-based devices targeting those on insulin therapy. Priced at $89 per month for a subscription or $99 for a single purchase of two sensors (each lasting up to 15 days), Stelo is positioned as a cost-effective alternative in a market where similar products can cost more than $250 per month. This move not only broadens Dexcom’s customer base but also addresses a previously underserved segment of the diabetes population—those managing their condition without insulin.
The Expanding Wearables Market
The wearables market has seen explosive growth in recent years, with global valuations expected to reach $265.4 billion by 2026. Health-focused devices, including CGMs, are a significant driver of this growth. Stelo’s introduction into the OTC market aligns with this trend, offering consumers a powerful tool for real-time glucose monitoring and insights without the need for a prescription.
This market expansion is further fueled by growing consumer interest in health and wellness, pushing companies to innovate and offer products that cater to both specific medical needs and general health monitoring. Stelo’s ability to provide continuous glucose readings and personalized health insights via a smartphone app places it at the forefront of this trend.
Competitive Dynamics and Market Shifts
The launch of Stelo is set to shake up the competitive landscape in the bio-wearables market. Abbott, a major player in this space, is also preparing to release its own OTC CGMs—Libre Rio for Type 2 diabetes management and Lingo for broader health and wellness monitoring. While Abbott’s Lingo is targeted more towards lifestyle management, Stelo is specifically aimed at individuals with Type 2 diabetes and pre-diabetes who are looking to better manage their condition.
Dexcom’s move into the OTC space not only challenges its competitors but also drives innovation in the market. The accessibility and affordability of Stelo may pressure other companies to lower prices or enhance their offerings to stay competitive. This increased competition is likely to benefit consumers through more choices, improved technology, and potentially lower costs.
Conclusion
Dexcom’s introduction of Stelo into the market is a significant milestone in both the CGM and broader wearables markets. By offering an affordable and accessible CGM without the need for a prescription, Dexcom is democratising diabetes management technology and empowering a wider audience to take control of their health. As the wearables market continues to evolve, products like Stelo are expected to play a crucial role in shaping the future of health monitoring, making advanced health management tools more commonplace and accessible to all. This move not only strengthens Dexcom’s market position but also sets the stage for a new era of health-focused wearables.
Comprehensive Support for Strategic Decision-Making
Empower your team with essential tools for informed decisions in dynamic market environments.
Get in touch with us
Please provide your details and we’ll contact you